Subject :Chemistry Class : Class 7
Subject :Chemistry Class : Class 10
Post Your Answer
Subject :Chemistry Class : Class 8
Post Your Answer
Subject :Chemistry Class : Class 7
Post Your Answer
Subject :Chemistry Class : Class 7
Post Your Answer
Subject :Chemistry Class : Class 6
Post Your Answer
Subject :Chemistry Class : Class 8
Post Your Answer
Subject :Chemistry Class : Class 6
Post Your Answer
Subject :Chemistry Class : Class 6
Post Your Answer
Subject :Chemistry Class : Class 6



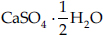 is called plaster of Paris.
is called plaster of Paris.