Subject :Chemistry Class : Class 9
Post Your Answer
Subject :Chemistry Class : Class 7
Post Your Answer
Subject :Chemistry Class : Class 7
Post Your Answer
Subject :Chemistry Class : Class 6
Match column I with column II and select the correct option from the given codes.
| Column I | Column II | ||
|---|---|---|---|
| (i) | Ginning | (A) | Cleaning and disentangling the fibres |
| (ii) | Combing | (B) | Brushing and straightening of fibres |
| (iii) | Carding | (C) | Bolls |
| (iv) | Seed pods | (D) | Cotton fibres after being separated from seeds |
| (v) | Lint | (E) | Separating fibres from seeds |
A(i) - (E); (ii) - (B); (iii) - (A); (iv) - (C); (v) - (D)
B(i) - (D); (ii) - (E); (iii) - (B); (iv) - (A); (v) - (C)
C(i) - (E); (ii) - (A); (iii) - (B); (iv) - (C); (v) - (D)
D(i) - (D); (ii) - (A); (iii) - (B); (iv) - (C); (v) - (E)
Post Your Answer
Subject :Chemistry Class : Class 7
Post Your Answer
Subject :Chemistry Class : Class 10
Post Your Answer
Subject :Chemistry Class : Class 8
Post Your Answer
Subject :Chemistry Class : Class 7
Post Your Answer
Subject :Chemistry Class : Class 7



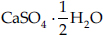 is called plaster of Paris.
is called plaster of Paris.